Heat exchanger protection with electrocoating
Corrosion, the deterioration of metals and alloys through a physical and/or chemical reaction with the environment, is expensive. Heat exchangers used in HVAC equipment, specifically condenser and cooling/heating coils are exposed in the environment, and this can lead to corrosion, failures, and performance degradation of the equipment in the cases of improper heat exchanger protection in corrosive locations.
Potentially corrosive environments include coastal and marine areas, locations adjacent to industrial and urban areas, locations with proximity to heavy road traffic, factories, power plants, or the combinations of the above.
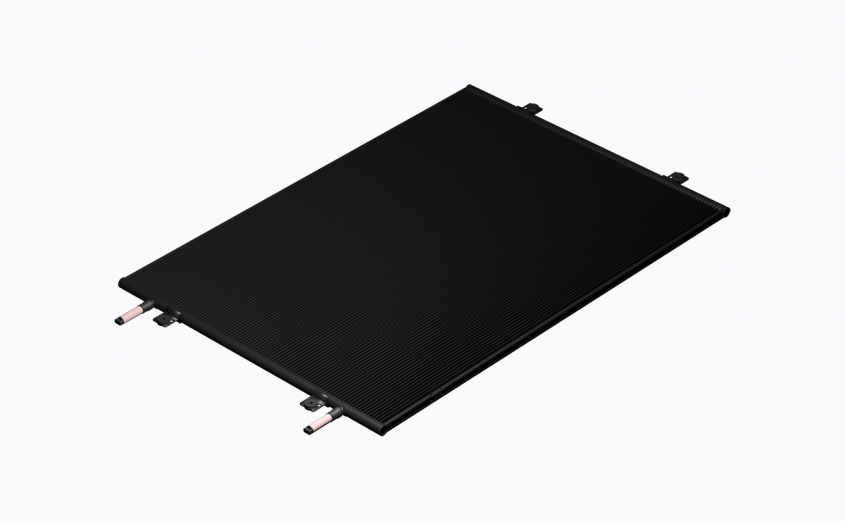
Unprotected heat exchangers, regardless of their type, are subjected to corrosion. Although all-aluminum microchannel coils tend to be less affected by corrosion compared to copper-aluminum finned-tube coils, the protection must be applied in order to prevent deterioration in aggressive atmospheres. The highest level of corrosion resistance can be achieved with the right coil coating. For microchannel heat exchangers, the best coating option is factory-applied electrodeposition, which is also referred to as electrocoating (e-coating) or electrophoretic deposition, and produce uniform finishing with excellent corrosion resistance.
Electrocoating Process
Electrocoating is a process based on the deposition of electrically charged particles out of a water suspension to coat a heat exchanger. During the electrocoat process, paint is applied to a heat exchanger with particular film thickness regulated by the amount of applied voltage and builds up an electrically insulating layer. The deposition process is self-limiting and stops as the applied coating electrically insulates the surface of a heat exchanger – thus guaranteeing substantial film thickness and complete surface coverage for such complex-shaped parts as microchannel heat exchangers.
Electrocoat process includes four distinct phases:
- Pre-treatment: cleaning the heat exchanger surface and phosphating. This stage includes immersion degreasing, rinsing, and phosphating, which is essential to achieving performance requirements and guarantees that no contaminations in the form of acids or electrolytes enter the electrocoat bath
- Applying the coating in an electrocoat bath. The bath is filling with paint emulsion (10-20%), solvents, and deionized water (80% and more), which is used as a carrier for the paint solids. The electrocoat process is driven by a DC rectifier, used to control the amount of paint that is deposited onto the heat exchanger surface. Cathodic deposition method with positively charged paint particles which are attracted to negatively charged heat exchanger characterizes by better corrosion resistance and high UV resistance of the end-product – compared to the anodic process. Thank electrical attraction, paint particles also penetrate the flaws and cracks in the metal
- Post-coating rinsing. Excessive paint is removed from the heat exchanger surface during this stage, providing a higher level of efficiency and aesthetics
- Thermal curing using bake oven. This process cures and cross-links the paint film to ensure maximum performance and corrosion resistance for the heat exchanger
Electrocoatings are typically made from polymeric resins, solvents and diluents, and pigments. Resin is a base of the paint which provides protection against corrosion and ultraviolet durability. Pigments and solvents provide coloring, glossing, and smooth appearance of the end product. The nature of the resins for the electrocoating can vary, but any type of resin feature functional groups in the backbone which allows them to become ionic in the presence of neutralizing agents.
Advantages Of Electrocoating
Electrocoating offers significant advantages over other coating technologies, including:
- High corrosion protection. Cathodic epoxy electrocoating with a film thickness of 20 microns withstands more than 1000 hours salt spray test performed in accordance with ASTM B117 standard
- Uniform coating with no more than 1-2 micron variances across the coated surface of any shape complexity
- Eco-friendliness: heavy metal free, no hazardous air pollutants (HAPS), low levels of organic solvents, and low volatile organic compounds (VOC)
- Aesthetic quality
Performance tests demonstrated the following results for e-coated microchannel heat exchangers manufactured by Kaltra:
|
TEST |
STANDARD |
RESULTS |
|---|---|---|
|
Dry film thickness |
ASTM D7091 |
15-50μm |
|
Film hardness |
ASTM D3363 |
>2H |
|
Adhesion rating |
ASTM D3359 |
0.0<ΔE<1.0 |
|
Salt spray test |
ASTM B117 |
6000hrs |
|
Water resistance in 100%rH |
ASTM D2247 |
>1000hrs |
|
Hot water dip test |
ASTM D870 |
>1000hrs |
|
Specular gloss test |
ASTM D523 |
60-90 |
|
Copper-accelerated acetic acid-salt spray test, CASS |
ASTM B368 |
>1000hrs |
|
Sea water acetic acid test, SWAAT |
ASTM G85 Annex A3 |
>3000hrs |
|
UV resistance test |
ASTM G154 |
>2000hrs |
Despite the additional cost of electrocoating, corrosion protection for heat exchangers is cost-effective: exponentially longer service life and failure-free equipment operation fully pay off all expenses related to electrocoating.